Why Are Only Valence Electrons Involved in Bonding
2 S has much weaker hydrogen bonding due to sulfurs lower electronegativity. Although Li has three electrons an isolated ground state Li atom does not yield Auger peaks because the atom has only two energy levels that contain electrons.
What Electrons Are Involved In The Most Types Of Bonding Quora
Auger peaks however have been detected from multiply excited Li atoms 7.
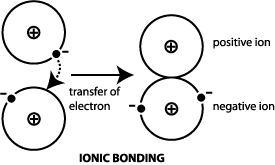
. This high heat capacity makes water a good heat storage medium. The extra bonding between water molecules also gives liquid water a large specific heat capacity. The presence of electrons in the valence band of solid Li also allows for Auger transitions of the type KVV.
H 2 S is a gas at room temperature despite hydrogen sulfide having nearly twice the molar mass of water.

Ces Information Guide Materials Science Engineering

The Electrons In The Outermost Energy Level Of An Atom At Level
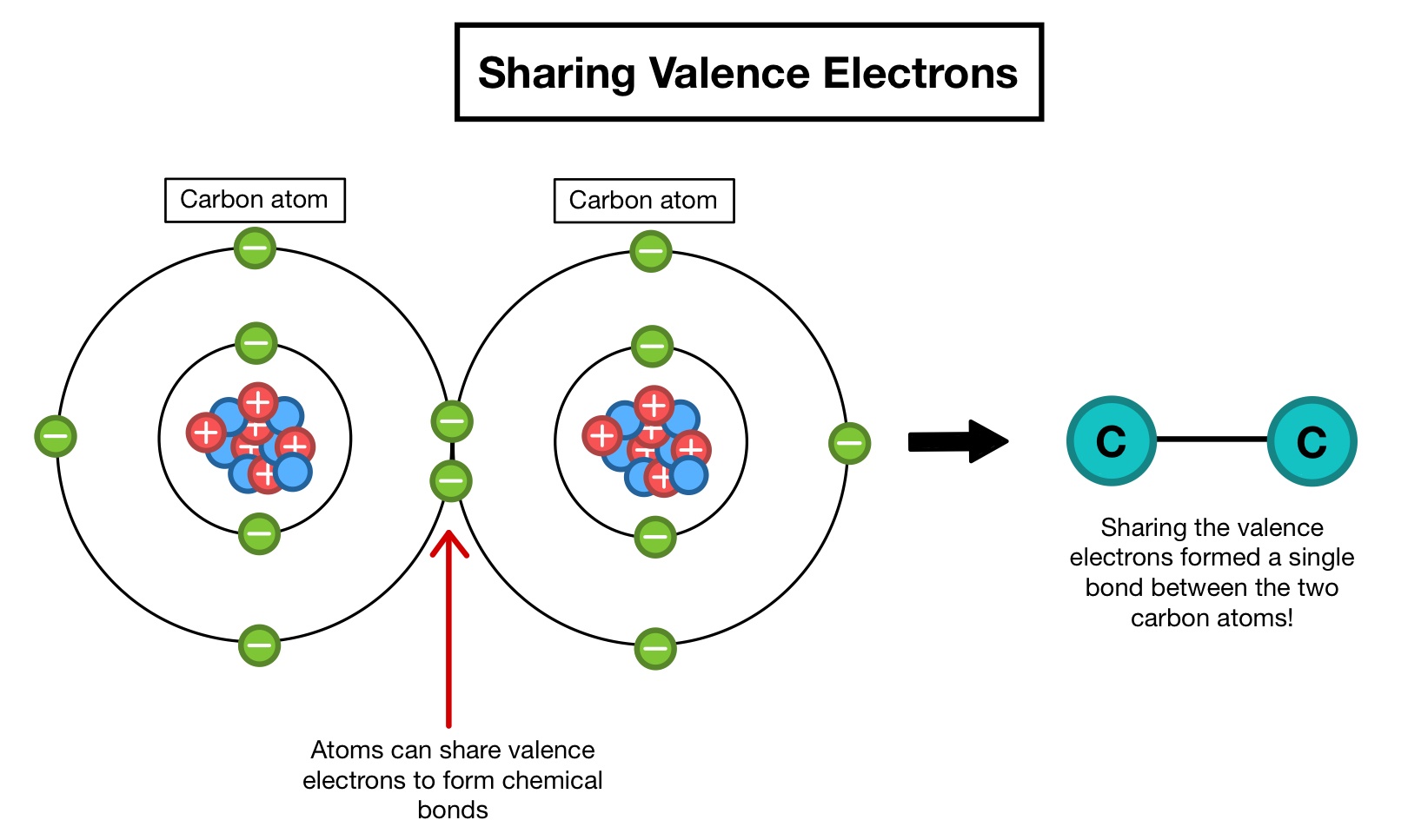
0 Response to "Why Are Only Valence Electrons Involved in Bonding"
Post a Comment